
The global climate change crisis combines the impact of excessive use of fossil fuels. Replacing carbon-based fossil fuels with clean energy harvested from renewable resources (solar, wind, tidal, geothermal, and so on) is the only viable long-term solution to address climate change. Due to the intermittent nature of renewable energies, energy-storage solutions should be implemented. Lithium-ion batteries (LIBs) are one of the most promising technologies. It’s really an idea for energy sustainability.
Rechargeable lithium-ion batteries are based on manganese oxide electrode materials which are more environment-friendly than conventional ones but generally suffer from rapid performance fading. A recent study sheds light on possible remedies through engineering of the interface.

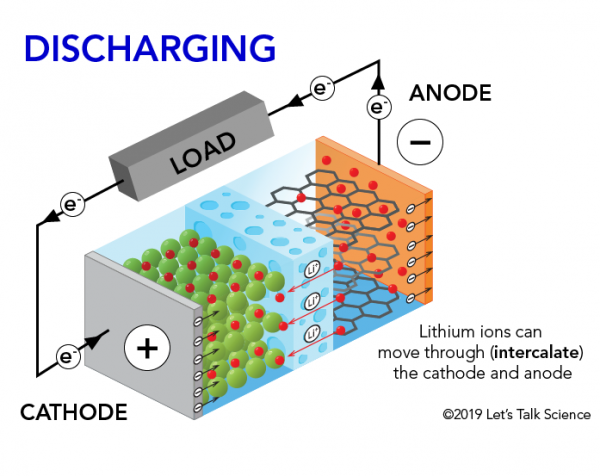
A lithium-ion (Li-ion) battery is an advanced battery technology that uses lithium ions as a key component of its electrochemistry. During a discharge cycle, lithium atoms in the anode are ionized and separated from their electrons. The lithium ions move from the anode and pass through the electrolyte until they reach the cathode, where they recombine with their electrons and electrically neutralize. The lithium ions are small enough to be able to move through a micro-permeable separator between the anode and cathode. Because of lithium’s small size, Li-ion batteries are capable of having a very high voltage and charge storage per unit mass and unit volume.
INNER STRUCTURE OF LITHIUM-ION BATTERY:-
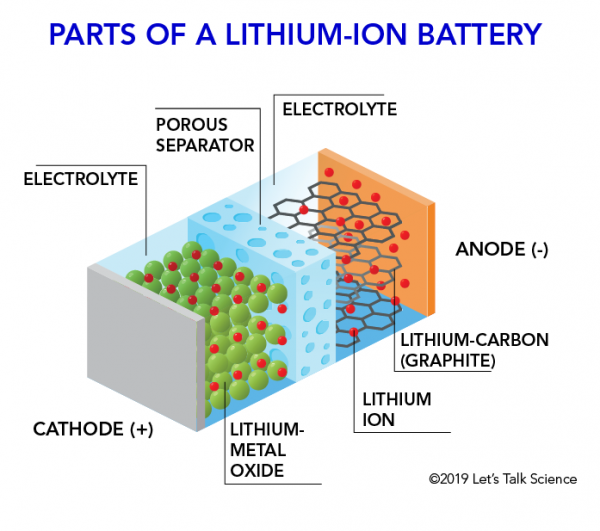
WORKING PRINCIPLE OF LITHIUM-ION BATTERIES:-
Inside a lithium-ion battery, oxidation-reduction (Redox) reactions take place.
Reduction takes place at the cathode. There, cobalt oxide combines with lithium ions to form lithium-cobalt oxide (LiCoO2). The half-reaction is:
CoO2 + Li+ + e– → LiCoO2
Oxidation takes place at the anode. There, the graphite intercalation compound LiC6 forms graphite (C6) and lithium ions. The half-reaction is:
LiC6 → C6 + Li+ + e–
Here is the full reaction (left to right = discharging, right to left = charging):
LiC6 + CoO2 ⇄ C6 + LiCoO2
- Basic Principle and Procedure of Fungal Staining
- What are Oxygen absorbers and How works?
- What is Pasteurization?
- What is Disinfectant and antiseptic? Disinfectants vs antiseptic
- Why More Men are Dying from Coronavirus than Women?
PROCESS of LITHIUM-ION BATTERIES:-
When the lithium-ion battery in your mobile phone is powering it, positively charged lithium ions (Li+) move from the negative anode to the positive cathode. They do this by moving through the electrolyte until they reach the positive electrode. There, they are deposited. The electrons, on the other hand, move from the anode to the cathode.
When you charge a lithium-ion battery, the exact opposite process happens. The lithium ions move back from the cathode to the anode. The electrons move from the anode to the cathode.
When you charge a lithium-ion battery, the exact opposite process happens. The lithium ions move back from the cathode to the anode. The electrons move from the anode to the cathode.
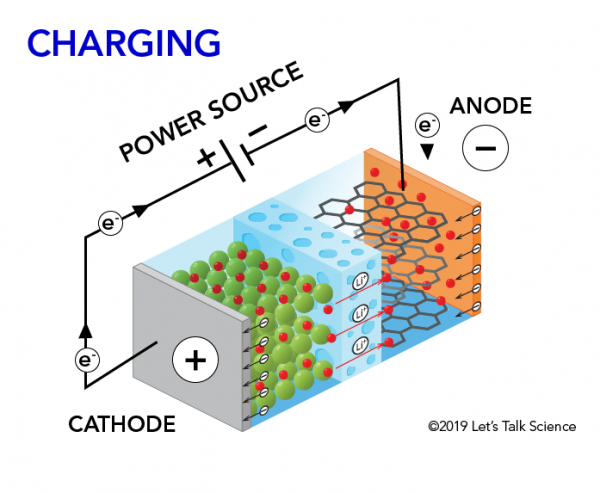
As long as lithium ions are making the trek from one electrode to another, there is a
constant flow of electrons. This provides the energy to keep our device running.
Since this cycle can be repeated hundreds of times, this type of battery is rechargeable.
ADVANTAGES:-
Li-ion batteries can use a number of different materials as electrodes. The most common combination is that of lithium cobalt oxide (cathode) and graphite (anode), which is most commonly found in portable electronic devices such as cell phones and laptops. Other cathode materials include lithium manganese oxide (used in hybrid electric and electric automobiles) and lithium iron phosphate. Li-ion batteries typically use ether (a class of organic compounds) as an electrolyte.
Compared to the other high-quality rechargeable battery technologies (nickel-cadmium or nickel-metal-hydride), Li-ion batteries have a number of advantages. They have one of the highest energy densities of any battery technology today (100-265 Wh/kg or 250-670 Wh/L).
In addition, Li-ion battery cells can deliver up to 3.6 Volts, 3 times higher than technologies such as Ni-Cd or Ni-MH. This means that they can deliver large amounts of current for high-power applications, which has Li-ion batteries are also comparatively low maintenance and do not require scheduled cycling to maintain their battery life.
Li-ion batteries have no memory effect, a detrimental process where repeated partial discharge/charge cycles can cause a battery to ‘remember’ a lower capacity. This is an advantage over both Ni-Cd and Ni-MH, which display this effect. Li-ion batteries also have a low self-discharge rate of around 1.5-2% per month. They do not contain toxic cadmium, which makes it easier to dispose of than Ni-Cd batteries.
Due to these advantages, Li-ion batteries have displaced Ni-Cd batteries as the market leader in portable electronic devices (such as smartphones and laptops). Li-ion batteries are also used to power electrical systems for some aerospace applications, notable in the new and more environmentally friendly Boeing 787, where weight is a significant cost factor. From a clean energy perspective, much of the promise of Li-ion technology comes from their potential applications in battery-powered cars. Currently, the bestselling electric cars, the Nissan Leaf and the Tesla Model S, both use Li-ion batteries as their primary fuel source.
Design/packaging of a LITHIUM-ION BATTERIES:
Li-ion cells (as distinct from entire batteries) are available in various shapes, which are generally divided into four groups:
- Small cylindrical (solid body without terminals, such as those used in older laptop batteries)
- Large cylindrical (solid body with large threaded terminals)
- Flat or pouch (soft, flat body, such as those used in cell phones and newer laptops; these are lithium-ion polymer batteries.
- Rigid plastic case with large threaded terminals (such as electric vehicle traction packs)
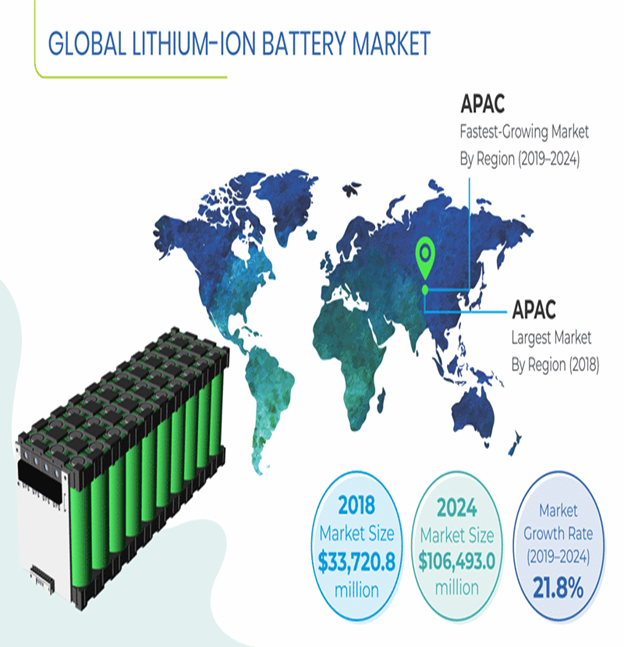
MARKET OUTLOOK OF LITHIUM-ION BATTERIES:-
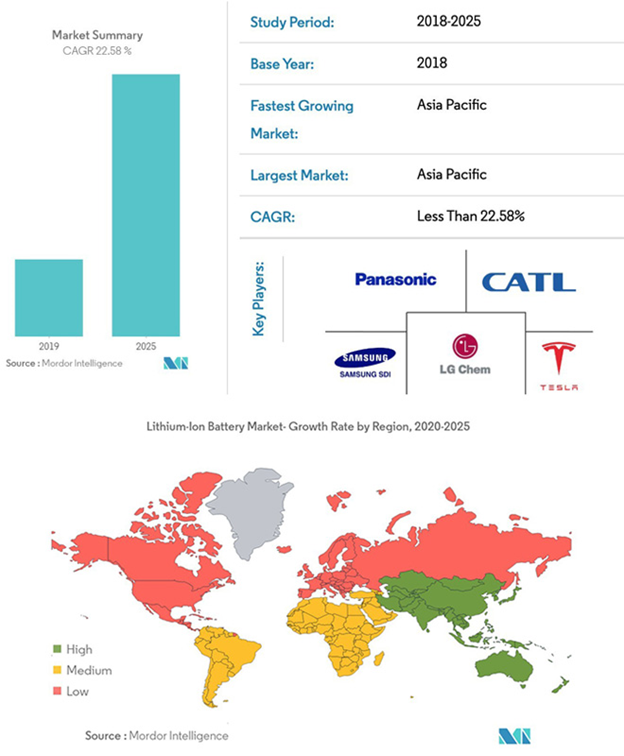
CONCLUSION:-
Currently, the lithium-ion battery deployment is anticipated to witness subdued growth on account of ongoing COVID-19 pandemic. Governments across the globe have implemented social distancing measures of varying degrees to control the spread of disease. Large scale lockdown of manufacturing units, and shortage of manpower are few of the factors that will decrement the installation of lithium-ion battery units.
However, if virus growth declines in the upcoming months, the industry statistics will witness accelerated growth due to the positive outlook toward renewable energy generation technologies and clean energy-based mobility solutions.
Author:

- Ransh Pharma: Best Intermediates Manufacturing Unit at Visakhapatnam
- What is 3D Bioprinting? Basic Principles, Techniques, and Application of 3D Bioprinting.
- Basic Principle and Procedure of Fungal Staining
- OXYGEN MANAGEMENT FOR SUSTAINABLE ENVIRONMENT
- mRNA VACCINES FOR COVID-19
- LITHIUM-ION BATTERIES An idea towards energy sustainability
- Artificial Leaf – A Blessing for Energy Crisis
- What are Oxygen absorbers and How works?
- What is Pasteurization?
- What is Disinfectant and antiseptic? Disinfectants vs antiseptic
- Why More Men are Dying from Coronavirus than Women?
- HOW COVID-19 SPREADS?
- What are the COVID-19 Symptoms?
- What is Coronavirus? How Coronavirus can Enter into our Body?
- Telemedicine Industry Analysis of Key Trends and Drivers Shaping Future Growth
- Rising Prevalence of Adverse Drug Reactions Drives Global Market for Pharmacovigilance
- Common Pharma Abbreviations
- Bioremediation : Promise for Eco-friendly enact|What is Bioremediation?
- GENERAL PROCEDURE FOR PASSIVATION
- How the New Drug approval process of CDSCO works?
- What is Passivation? How Does Passivation Process Work? How to Passivate Stainless Steel Parts?
- Explore new openings in Pharma Profession by Making yourself Skilled as Technical Associate in The Central Drugs Standard Control Organisation CDSCO
- What is Data Integrity and ALCOA Plus
- GOWNING PROCEDURE IN CLEAN ROOM
- How to Crack Your Job Interview?
- 10-Deacetylbaccatins (10 DAB) NATURAL ORGANIC COMPOUNDS USED AS STARTING MATERIAL FOR ANTICANCER DRUGS PRODUCTION
