
Oxygen Absorbers or Oxygen buster are used to remove oxygen from a sealed container, creating a nitrogen environment for long-term food/ pharmaceutical product storage.

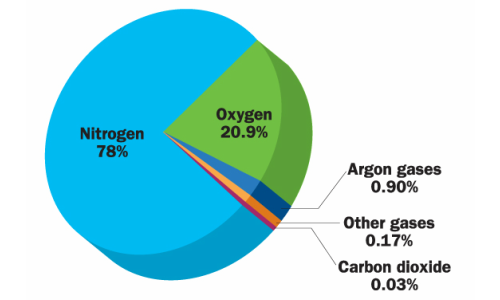
Because air contains more than 78% nitrogen, more than 20% oxygen, and 1-2% water vapor and other gases also present at a very minimal percentage. Only Oxygen supports the growth of microorganisms and Oxygen react with different chemical compound and causes changes in color and odors in packaged Pharmaceutical and food products.
What Are the Benefits of Using Oxygen Absorbers?
- Extends shelf life of Pharmaceutical and food Products
- Prevents the growth of aerobic pathogens/ Microorganisms and spoilage organisms, including molds.
- It can prevent oxidation and hydrolysis in pharmaceuticals and help protect oxygen-sensitive medical devices and equipment.
- Prevents oxidation of vitamins A, C, and E
What Are Oxygen Absorbers Made Of?
Oxygen buster is two types. one is iron content and another is iron-free.

Oxygen absorbers are small packets/ Medium Packets that contain iron dust or powder. The packets are made of a permeable membrane that allows oxygen and moisture to enter into the packet but does not allow the iron powder to leak out from the packet. The Oxygen buster is safe to place on top of the food. They will not harm the food they are in contact with as they come in a sealed pouch.

Another oxygen scavenger being an iron-free oxygen absorber. An iron-free oxygen absorber is made of Ascorbic Acid, Sodium Carbonate, Activated Carbon, Water, Diatomaceous Earth which do not allow oxygen and moisture to enter the product
How Do Oxygen Absorbers Work?
‘Oxygen absorbers’ perform their action though a chemical reaction. They contain iron powder which reacts with the oxygen in the air causing the iron powder to rust. When all the iron powder has oxidized, the “oxygen absorbers” are “loaded” and the absorbing action stops. Remove the oxygen from an active absorber and the chemical reaction stops. Put them back in the air and the reaction starts again until the iron is gone.
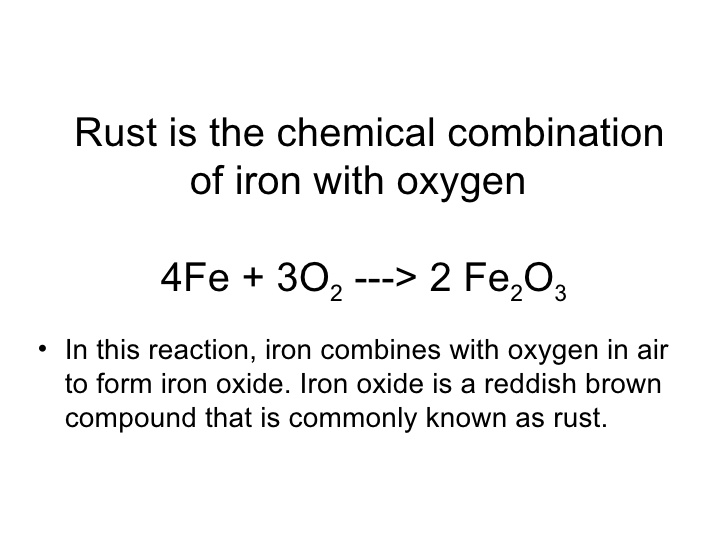
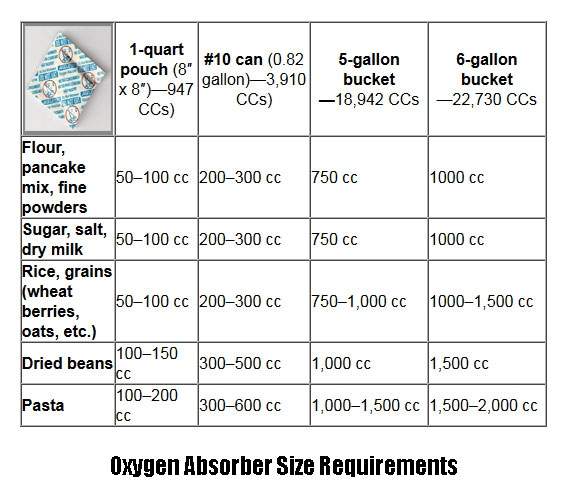
What Amount of Oxygen Absorber Should I Use?
Watch this video to know about oxygen Absorbers:
- Basic Principle and Procedure of Fungal Staining
- What are Oxygen absorbers and How works?
- What is Pasteurization?
- What is Disinfectant and antiseptic? Disinfectants vs antiseptic
- Why More Men are Dying from Coronavirus than Women?
- COVID-19 IN HIGH TEMPERATURE: WILL SUMMER TEMPERATURE KILL THE CORONA VIRUS?
- Pool Sample: ICMR Advises ‘New Testing Method’ to increase the number of COVID-19 Test
- করোনা ভাইরাস কি? (What is Corona Virus?) করোনা ভাইরাস (COVID-19) কিভাবে ছড়ায়?
- Why 70% of Alcohol (IPA) used for Hand Sanitizer?
- Social Distancing: What is Social Distancing? Does social distancing work?
- COVID-19 and Pregnancy: Risk of COVID-19 infection in Pregnant Women and New Born Baby
- What are the COVID-19 Symptoms?
- What is Coronavirus? (COVID-19) How Coronavirus can Enter into our Body?
- Vizag Gas Leak: Styrene Gas Leaked from LG Polymers
- Coronavirus (COVID-19) Vaccine: ChAdOx1, developed at Oxford’s Jenner Institute
- Use of Silver Nanoparticles in HIV Treatment
- Salt stress and Phyto-biochemical Responses of Plants (Review Article)
- RHEUMATOID ARTHRITIS AND ITS TREATMENT (A REVIEW ARTICLE)
