
Ransh Pharma is a well-known pharmaceutical organization. Ransh has been involved in the manufacturing of various Drug Intermediates and Key Starting Materials for APIs. We are selling our products in the domestic and export markets. We are constantly engaged in delivering quality products to our esteemed customers through innovative technologies, customer satisfaction, and services. Our…

In this article, you know about 3D Bioprinting. Means what is 3D Bioprinting? Basic Principles, Techniques, and Application of 3D Bioprinting. BIOPRINTING IS DEFINED AS THE POSITIONING OF BIOCHEMICALS, BIOLOGICAL MATERIALS, AND LIVING CELLS FOR THE GENERATION OF BIOENGINEERED STRUCTURES (I.E., ADDITIVE MANUFACTURING) OF BIOLOGICAL AND BIOLOGICALLY RELEVANT MATERIALS WITH THE USE OF COMPUTER-AIDED TRANSFER…
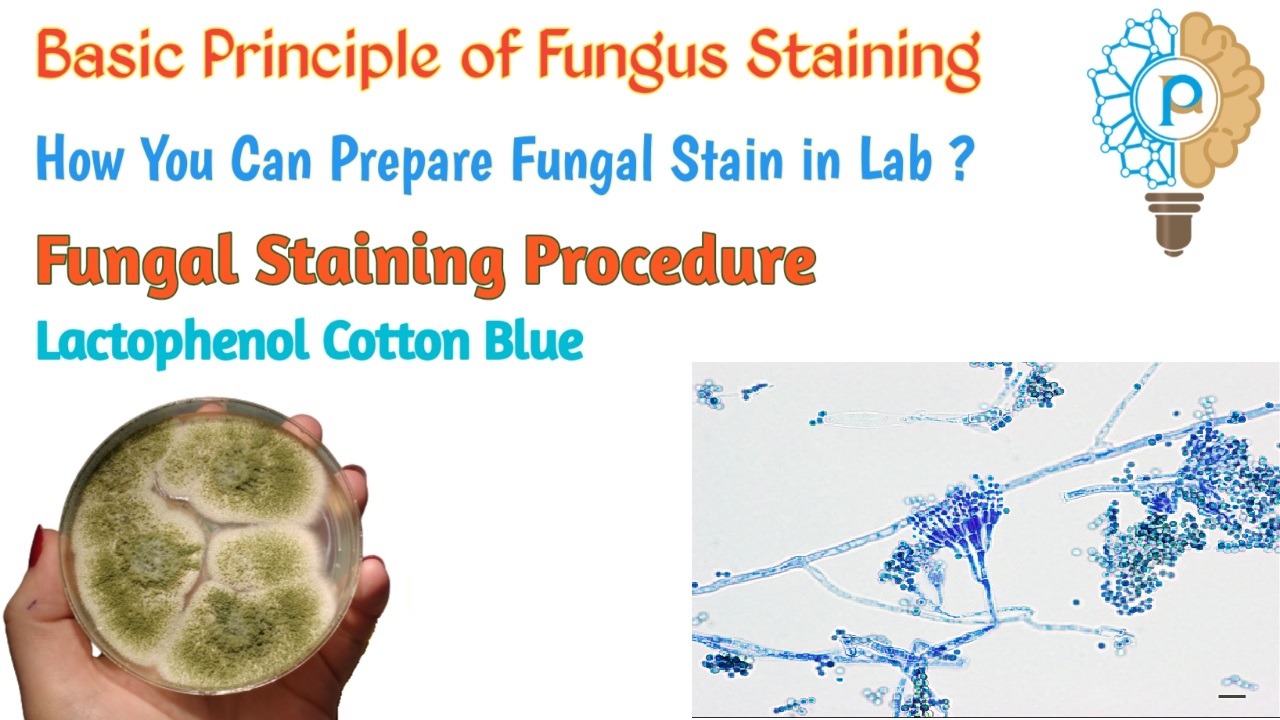
Fungal staining is a very short and easy method. Here you learn Basic Principle and Procedure of Fungal Staining. What is fungus? A fungus is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and molds, as well as the more familiar mushrooms. Fungi have cells with nuclei. Their cell walls…

An average human being consumes around 740-750 litres of oxygen per year, multiplied with the world’s population, total oxygen consumption only by human beings, I repeat, only by human beings, is close to 5.8 trillion litre per year. Interestingly, the air in Earth’s atmosphere is made up of approximately 78 percent nitrogen and 21 percent…
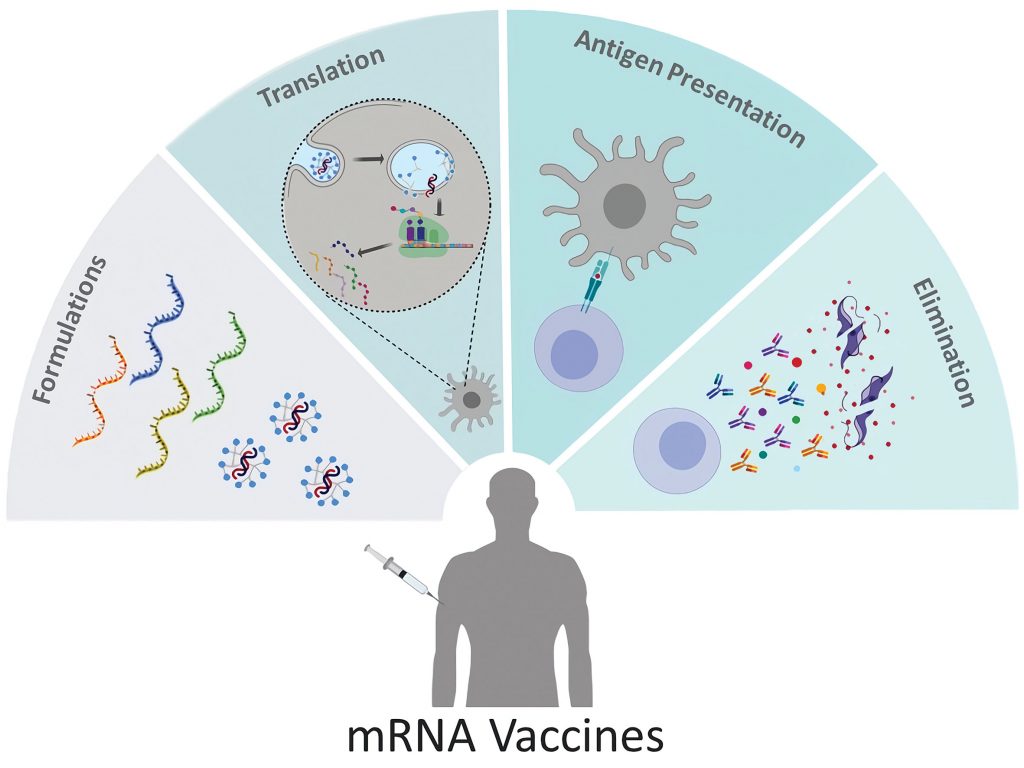
Vaccination is one of the major success stories of modern medicine, greatly reducing the incidence of infectious diseases. (measles, and eradicating others, such as smallpox). Conventional vaccine approaches have not been as effective against rapidly evolving pathogens like influenza or emerging disease threats such as the Ebola or Zika viruses. RNA-based vaccines could have an…

The global climate change crisis combines the impact of excessive use of fossil fuels. Replacing carbon-based fossil fuels with clean energy harvested from renewable resources (solar, wind, tidal, geothermal, and so on) is the only viable long-term solution to address climate change. Due to the intermittent nature of renewable energies, energy-storage solutions should be implemented….

The environmental and associated concerns including pollution, climate change, health issues, etc. All the environmental and associated concerns have drawn quite some attention globally over the years. While there is no mechanism to completely eliminate these challenges. A lot of efforts are being made worldwide to reduce the impact of these issues and build a…

Oxygen Absorbers or Oxygen buster are used to remove oxygen from a sealed container, creating a nitrogen environment for long-term food/ pharmaceutical product storage. Because air contains more than 78% nitrogen, more than 20% oxygen, and 1-2% water vapor and other gases also present at a very minimal percentage. Only Oxygen supports the growth of…

Pasteurization is a heat-treatment process that destroys pathogenic microorganisms in certain foods and beverages. The name comes from French Microbiologist Louis Pasteur. Louis Pasteur (in the 1860s) was demonstrated that abnormal fermentation of wine and beer could be prevented by heating the beverages to about 57° C (135° F) for a few minutes. The Purpose of…

Disinfectant and antiseptic are widely used in industries (Mainly Pharmaceuticals and Food industries) and as well as in our house. Antiseptics and disinfectants are also used in the medical and healthcare industries. Surgeons, for instance, scrub themselves with antiseptics before operating. Various healthcare workers wash their hands with antiseptics in hospitals. The purpose of the uses of both agents is…
| Powered by WordPress | Theme by TheBootstrapThemes