Airlocks are called PAL (Personnel Air Lock) when used for personal and MAL (Material Air Lock) when used for transferring the material.
2.0 Bubble Airlock
3.0 Sink Airlock
4.0 Potent compound airlock
These airlocks are very common having higher pressure on one side and lower pressure on another side. In this system, positive air pressure flows from the higher pressure internal zone to be airlock and from the airlock to the lesser lower pressure grade area. This prevents entry dust and contamination from outside to airlock and from airlock to inner side.
Any manufacturing facilities where the product requires protection from particulate but the people outside the clean-room do not need protection from the product in the clean room.
2.0 Bubble Airlock:
These types of airlock having higher pressure inside the airlock and lower pressure both outside. As it runs at positive pressure to both areas it creates a barrier where contaminants within either area are pushed back into their own respective areas.
Application:
Used in, areas where the product needs protection and the people external to the clean rooms require protection from the product, to reduce the possibility of viable articulate from entering the lesser pressure clean-room. Area such as higher potency, compounding areas terminal sterilization is not an option.
3.0 Sink Airlock:
Airlocks having lower pressure inside the airlock and higher pressure on both sides of the airlock. This airlock pulls air from both adjacent areas creating a low-pressure barrier and reduces the opportunity of contamination passing to the internal zone.
Application:
In many research facilities, substances that are experimented on are highly dangerous, and it is essential to keep them from being exposed. During a few types of production processes in a clean-room, air from a contaminated area has to be contained in one place.
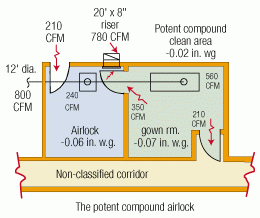
4.0 Potent compound airlock:
The “potent compound airlock” is a combination of the pressure bubble and pressure sink airlocks. This two-compartment airlock arrangement allows personnel to protect (gown/respirator) themselves before coming into contact with any dangerous materials while at the same time, the product (potent compound) is protected from contamination from adjacent, connected areas. All conditioned, clean air supplied to the gown room is dissipated into the adjacent rooms while all the conditioned, clean air supplied to the airlock room (as well as all infiltration air into that room) is exhausted.
Pharmaceutical and Biopharmaceutical industries are meant to treat diseases and also give immunity to patients from dangerous diseases. A proper design must ensure that clean and sterile products are produced preventing any reintroduction of bacteria or allergens or any disease-causing materials into the systems, materials, and process.
A proper URS and Subject matter expertise is the need of the hour to design, Qualify and operate such Clean room facilities with good airlocks, In one say we would call Air Locks are ventricles of the heart. If they fail the whole system collapses.
1. ISPE Baseline Pharmaceutical Guide Volume 3 – Sterile Manufacturing Facilities First Edition/January 1999. ISPE, 3816 W. Linebaugh Ave. Suite 412, Tampa, FL 33624; Phone: (813) 960-2105; Fax: (813) 264-2816.
2. 1999 ASHRAE Handbook – HVAC Applications American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc.; 1791 Tullie Circle, N.E., Atlanta, GA 30329; (404) 636-8400.
3. Guideline on Sterile Drug Products Produced by Aseptic Processing, Center for Drugs and Biologics, Food and Drug Administration, Rockville, MD, June 1987.
4. www.pharmatech.com
More Article for You
- What is the fumigation and fogging?
- What is viable and nonviable Particle Count?
- Scope for Science Graduate & Post Graduate
- Types of Clean Room Airlocks in cGMP Facility
- Simplified CAPA with Investigation Tools, Route Cause Analysis & Risk Management
- KNOW ABOUT TOTAL QUALITY MANAGEMENT AND CURRENT GOOD MANUFACTURING PRACTICES (cGMP)
- Difference between C8 and C18 Columns Used in HPLC System
- How to Crack Your Job Interview?
- GOWNING PROCEDURE IN CLEAN ROOM