About Author:
Mr. Shoab Malek
(Certified Quality Auditor)
Degree Required:-
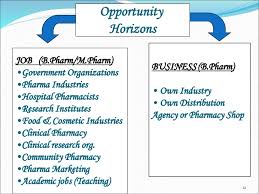
B.Sc :- Bachelor of Science with subject of Chemistry, Microbiology, Biotechnology & Zoology can work in pharmaceutical, life science, Biologicals & Medical device industry as a Trainee in Production, QC, Micro Or Warehouse Department. Some Clinical Research Organization (CRO) are also hired come B.Sc Fresher as a Custodian, Phlebotomist or as a Research Assistant in Bio-analytical department.
D.Pharm / B.Pharm:- Pharmacy Graduate candidate are eligible to work in Production, QC & QA department. In CRO Pharmacy Graduates can work as a Clinical Research department, Documentation, QA, & in Bio-analytical department.
M.Sc :– Master of Science with subject of chemistry, Microbiology, Biotechnology & Zoology are eligible to work in Production, QC, QA, Micro & warehouse department. In CRO M.Sc fresher are eligible to work in Clinical research, QA, Documentation & Bio-analytical department.
M.Pharm :– Masters in Pharmacy candidate are highly demanded as of now and they are eligible to work in all department and all field expect Microbiology Laboratory. In CRO Pharmacy Graduates can become a Reviewer, Auditor, Analyst, Chemist and Clinical research Professional.
Industry Identification:-
1) Pharmaceuticals :-
· Pharmaceuticals industry divided in five production division such as
1) Oral Solid Dosage Form (Tablet & Capsule)
2) Liquid Dosage Form (Large Volume Parental)
3) Injection (Sterile Small Volume parental)
4) Ointment / Ophthalmic ( In Semi Solid dosage form either sterile or non Sterile)
5) Cephalosporin / Beta lactum dosage form in Dry powder, OSD or Injectable. (Sterile & Non Sterile Both.)
· In this manufacturing process of Oral Dosage Form (Tablet & Capsule),
Ø Dispensing of Raw Material / Packing Material,
Ø Shifting – Mixing – Paste addition – Drying – Milling & Final Mixing with Lubrication
Ø After QC release of this Final Mixing granules process may go ahead with
Ø Compression
Ø Coating (If Required)
Ø Visual Inspection
Ø Primary packing / Bulk Packing
Ø Secondary Packing
Ø Dispatch
· QC Department: – To perform analysis of different stage samples like Blend Sample (In Process), Compression Sample (In Process), Coating (In Process) & finished product sample during packing.
· Micro Department: – To perform microbial analysis of above defined samples, perform Environment Monitoring.
· Warehouse Department: – To Dispense Raw Material & Packing Material as per Production / QA Requisition.
· QA Department :- QA Department are involved in each & every step & Process. In Process QA personnel are responsible for Dispensing, All stage in process checks with online documentation. QMS & Validation QA Personnel are responsible for Validation & Quality Management Activity.
· Dispatch of finished goods is also carried out in presence of QA.
· In this manufacturing process of Liquid Parental (Syrup Preparation),
Ø Dispensing of Raw Material / Packing Material,
Ø Syrup Preparation —— Filtration through Basket Filter, (Continuous Stirring & Mixing)
Ø Addition of Active Pharma Ingredient ——- Addition of Flavour & Color. (Continuous Stirring & Mixing)
Ø Final Mixing at high Speed ——- Filtration —— Transfer Storage Tank
Ø After QC release process may go ahead with
Ø Bottle Washing & Drying
Ø Liquid (Syrup) Filling
Ø Capping / Sealing
Ø Visual Inspection
Ø Labeling & Packing
Ø Dispatch
· QC Department: – To perform analysis of different stage samples like Final Mixed after Filtration Sample (In Process), During filling (In Process) & finished product sample during packing.
· Micro Department: – To perform microbial analysis of above defined samples, perform Environment Monitoring.
· Warehouse Department: – To Dispense Raw Material & Packing Material as per Production / QA Requisition.
· QA Department: – QA Department are involved in each & every step & Process. In Process QA personnel are responsible for Dispensing, All stage in process checks with online documentation. QMS & Validation QA Personnel are responsible for Validation & Quality Management Activity.
· Dispatch of finished goods is also carried out in presence of QA.
· In this manufacturing process of Small Volume Parental (Injection), (Fully Sterile Operation)
Ø Dispensing of Raw Material / Packing Material,
Ø Take Water for Injection (WFI) in Manufacturing tank———Addition of raw material including API with starring & mixing
Ø Volume make up and Final Mixing —— Filtration ——- Transfer in storage tank
Ø After QC release process may go ahead with
Ø Ampoule / Vial Washing
Ø Sterilization of washed Ampoule / Vial (By Dry Heat Sterilizer or Under High temperature Tunnel)
Ø Filling of Bulk
Ø Capping / Sealing
Ø Visual Inspection
Ø Labeling & Packing
Ø Dispatch
· QC Department: – To perform analysis of different stage samples like Final Mixed after Filtration Sample (In Process), During Filling (In process) & finished product sample during packing.
· Micro Department: – To perform microbial analysis of above defined samples, perform Environment Monitoring. (Check Sterility)
· Warehouse Department: – To Dispense Raw Material & Packing Material as per Production / QA Requisition.
· QA Department: – QA Department are involved in each & every step & Process. In Process QA personnel are responsible for Dispensing, All stage in process checks with online documentation. QMS & Validation QA Personnel are responsible for Validation & Quality Management Activity.
· Dispatch of finished goods is also carried out in presence of QA.
·In this manufacturing process of Ointment / Ophthalmic (Sterile & Non Sterile Operation),
Ø Dispensing of Raw Material / Packing Material,
Ø Preparation of Wax in wax preparation vessel ——– Transfer Wax in manufacturing vessel
Ø Addition of Raw Material & API in wax with continuous mixing & stirring————-Final Mixing at high speed.
Ø After QC release process may go ahead with
Ø Empty Tube Arrangement
Ø Tube Filling
Ø Labeling & Embossing
Ø Weight Checking
Ø Packing
Ø Dispatch
· QC Department: – To perform analysis of different stage samples like Final Mixed Sample (In Process), During Filling (In process) & finished product sample during packing.
· Micro Department: – To perform microbial analysis of above defined samples, perform Environment Monitoring. (Check Sterility)
· Warehouse Department: – To Dispense Raw Material & Packing Material as per Production / QA Requisition.
· QA Department: – QA Department are involved in each & every step & Process. In Process QA personnel are responsible for Dispensing, All stage in process checks with online documentation. QMS & Validation QA Personnel are responsible for Validation & Quality Management Activity.
· Dispatch of finished goods is also carried out in presence of QA.
Note: – For Cephalosporin & Beta Lactum Manufacturing of OSD same process and manufacturing followed as I described and for Injection and Dry Powder Filling also same process follows as Small Volume Parental.
2) Life science / Bioscience:- (Fully Sterile Operation)
· Life sciences / Bioscience industry involved in manufacturing of Vaccine,
1) Poultry Vaccine
2) Animal Vaccine
3) Human Vaccine
· In Vaccine Production Process flows as are follows,
Ø Receiving of SPF (Specific Pathogen Free) Eggs in facility
Ø Inoculation of Antigen / Virus in Eggs
Ø Incubation of Eggs in incubator for 7-14 days
Ø On each day perform Candle test of all Inculcated eggs. (If any egg found dead than remove it)
Ø If Vaccine is Fluid based than collect fluid and discard embryo
Ø If Vaccine is Embryo based than remove fluid and collect embryo
Ø In case of fluid based vaccine use fluid directly in Blending
Ø In case of Embryo based vaccine collect embryo than crush in Silverson jar & Than Mixer and then filter this fluid
Ø After getting fluid this fluid in Blending
Ø During Blending add stabilizing agent and required fluid and mix it properly
Ø Than Filter this blend
Ø Filling of Fluid
Ø Either lyophilized (Live vaccine) or Liquid form (Killed Vaccine)
Ø Visual inspection
Ø Labeling & Packing
Ø Dispatch
· QC Department: – To perform analysis of different stage samples like Blend Sample (In Process), Filling Sample (In Process) & finished product sample during packing.
· Micro Department: – To perform microbial analysis of above defined samples, perform Environment Monitoring. (Check Sterility)
· Warehouse Department: – To Dispense Raw Material & Packing Material as per Production / QA Requisition.
· QA Department: – QA Department are involved in each & every step & Process. In Process QA personnel are responsible for Dispensing, All stage in process checks with online documentation. QMS & Validation QA Personnel are responsible for Validation & Quality Management Activity.
· Dispatch of finished goods is also carried out in presence of QA.
3) Biologics:-
· Biologics industry involved in manufacturing of Biologics Product,
1) Hormones
2) Plasma Proteins
3) Monoclonal Antibody
4) Biosimilars
5) Noval Proteins
· In Vaccine Production Process flows as are follows,
Ø Centrifugation of (Purification) of Blood in Plasma & Serum
Ø Collect Plasma in separate container / Bag
Ø For Purification process prepare different Buffers (Pre equilibrium, Equilibrium & Post equilibrium)
Ø Purification of Required plasma protein by Column Chromatography
Ø By using different buffers purify plasma protein
Ø Collect purified plasma protein in cleaned container / tank
Ø Adjust ph as required
Ø Filter this plasma protein by assembly filter (5 micron to 0.2 micron).
Ø Filling of Plasma protein in Bulk or Bottle as required.
Ø Visual inspection
Ø Labeling & Packing
Ø Dispatch
· QC Department: – To perform analysis of different stage samples like Blend Sample (In Process), Filling Sample (In Process) & finished product sample during packing.
· Micro Department: – To perform microbial analysis of above defined samples, perform Environment Monitoring. (Check Sterility & BET)
· Warehouse Department: – To Dispense Raw Material & Packing Material as per Production / QA Requisition.
· QA Department: – QA Department are involved in each & every step & Process. In Process QA personnel are responsible for Dispensing, All stage in process checks with online documentation. QMS & Validation QA Personnel are responsible for Validation & Quality Management Activity.
· Dispatch of finished goods is also carried out in presence of QA.
4) Biotech:-
· Biotech industry involved in manufacturing of API & Excipients ( Raw Material)
· Active Pharmaceutical Ingredients (API) are Fermentation based process
· Even Excipients are just mixing process of different ingredients
· In API Production Process flows as are follows,
Ø Dispensing of Raw Material
Ø Preparation of Salt (Buffer) (Small Fermenter)
Ø Preparation of Media (Small Fermenter)
Ø Inoculation of Micro organisms of required species in Media
Ø Mixing of Materials in Manufacturing tank (Large Scale Fermenter)
Ø Addition of Salt (Buffer) in Manufacturing tank
Ø Transfer Media in Manufacturing tank
Ø Mixing of Final product till good & required growing of micro organisms
Ø Continuous sampling during mixing for in process checks
Ø After getting good growth of organisms stop mixing
Ø Transfer this product in Reactor
Ø Collect & store it with IPR or required solvent for required time
Ø Belding & Final mixing in Blender
Ø Packing
· QC Department: – To perform analysis of different stage samples like Mixing (In Process), & finished product sample during packing.
· Micro Department: – To perform microbial analysis of above defined samples, perform Environment Monitoring.
(Check Sterility & BET if Required)
· Warehouse Department: – To Dispense Raw Material & Packing Material as per Production / QA Requisition.
· QA Department :- QA Department are involved in each & every step & Process. In Process QA personnel are responsible for Dispensing, All stage in process checks with online documentation. QMS & Validation QA Personnel are responsible for Validation & Quality Management Activity.
· Dispatch of finished goods is also carried out in presence of QA.
5) Medical Device:-
· Medical Device industry involved in manufacturing of multiple product, (Sterile & non Sterile both)
· They are involved in production of multiple products are like Syringe, IV set, Blood Bag, Sterile Gown, Nose, Mask, Sterile Wears etc….
· In Medical Device Production Process flows as are follows,
Ø Collection of required different size product parts or assembly
Ø In case of Syringe Plastic molding shall be in house.
Ø Washing & drying of different parts and assembly under controlled environment.
Ø Fixing of Assembly or product parts by lock & key
Ø Final Washing & Drying of Product
Ø Packing & Labeling of Product
Ø ETO Sterilization of products in bulk.
Ø Dispatch
· QC Department: – To perform analysis of different stage samples like (In Process) & finished product sample during packing.
· Micro Department: – To perform microbial analysis of above defined samples, perform Environment Monitoring. (Check Sterility & BET)
· Warehouse Department: – To Dispense Raw Material & Packing Material as per Production / QA Requisition.
· QA Department :- QA Department are involved in each & every step & Process. In Process QA personnel are responsible for Dispensing, All stage in process checks with online documentation. QMS & Validation QA Personnel are responsible for Validation & Quality Management Activity.
· Dispatch of finished goods is also carried out in presence of QA.
6) Clinical Research Organization:-
· Clinical research industry involved in Clinical Research on Humans in different phases like Phase I , Phase II, Phase III & Phase IV
· These Clinical trials are executed under continues monitoring of highly experienced Doctors & Clinical Research professional.
· During Clinical trials Continuous sampling and analysis shall be performed in Bio-analytical department and monitoring of patient is also continuously checked.
· In Clinical Research Process flows as are follows,
Ø Selection of healthy volunteer by mutual agreement
Ø Selected volunteers are admitted in house hospitals.
Ø Drugs are injected in volunteers body
Ø Under Continuous monitoring sampling of blood shall be done
Ø This blood are collected by Phlebotomist & this blood sent it to Bio analytical department.
Ø By using Centrifugation method blood shall be purified in Plasma & Serum.
Ø Plasma Samples are stored in clod room with proper labeling.
Ø These samples are analyzed on HPLC – MS or UPLC –MS System.
Ø By reviewing results study can be extended to next phase.
· Bio Analytical Department: – To perform analysis of different stage samples like.
· QA Department: – QA Department are involved in each & every step & Process. All stage checks with online documentation. QMS & Validation QA Personnel are responsible for Validation & Quality Management Activity.
· Continuous audit of Clinical research & Bio analytical monitoring shall be performed by QA Personnel.
· Clinical research & Bio analytical testing data shall be reviewed.
Government Job:-
· Science Graduate or Post Graduate students can get Job in multiple government sectors.
· By clearing individual exams for teacher, lecturer, state government competitive exams, and Central Competitive exams.
· Science Graduate or Post Graduate students are also eligible for Bank exams.
· In different sectors of healthcare science graduate can get Job as Lab. Assistant, Health Inspector, Pharmacist, Microbiologist, Compounder etc.
Note:-
· All information in this documents are based on my personal knowledge and experience so, it might be wrong or not complete.
· This document is not based on particular company or industry but it is just for rough idea for Science Graduate Fresher’s.
· I am not concern to affect any industry or Company so all readers do not take this information as complete & right as source.
Please follow and like us: